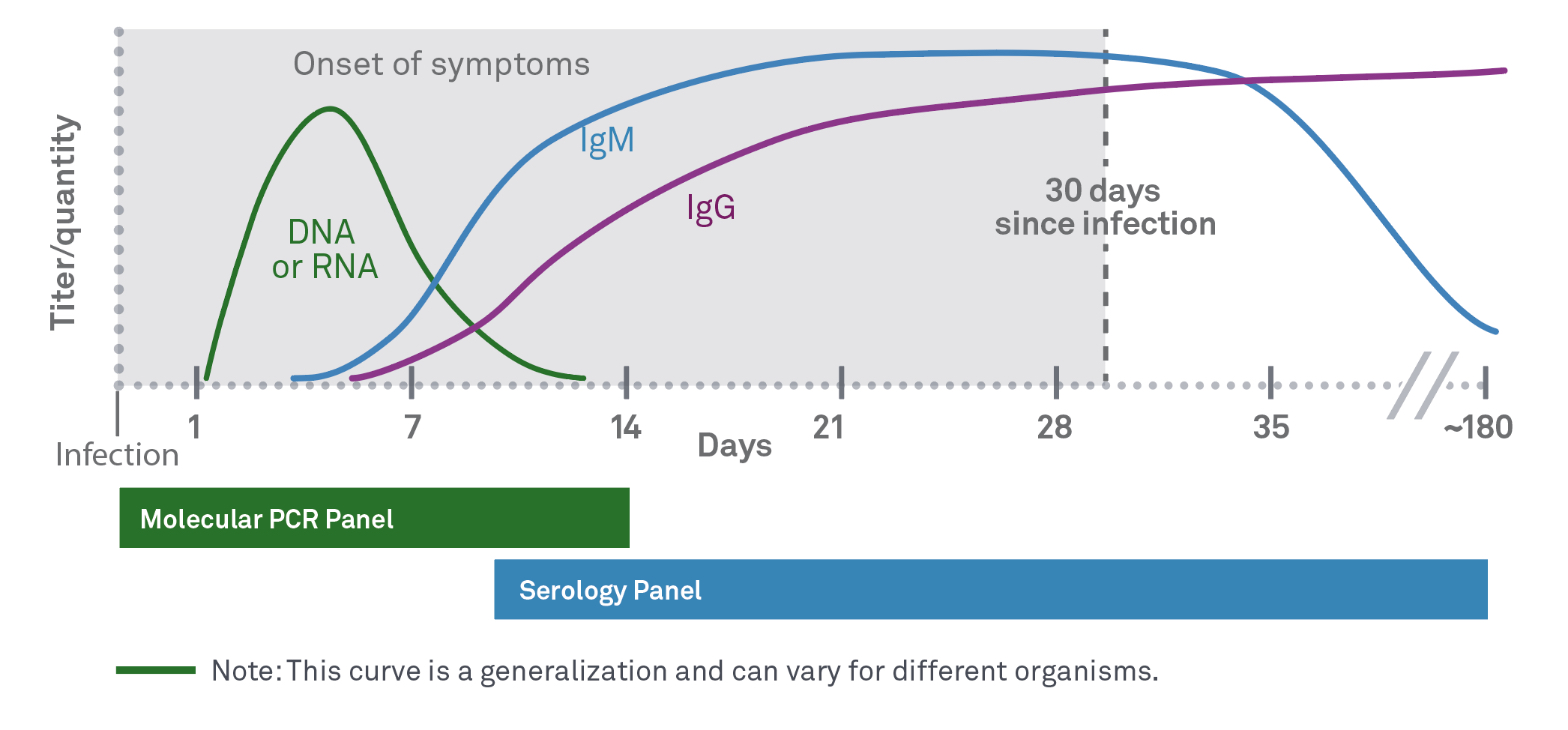
- IgM antibodies in some individuals are known to persist for much longer than 2-3 months
- CDC does not recommend testing for IgM with STTT if signs or symptoms exceed 30 days
|
Test code |
36942 |
|
Preferred specimen |
2 mL serum collected in a serum separator tube (SST) and transferred to a plastic transport tube |
|
Turnaround time |
1-3 days |
Panel components:
|
TEST CODE |
TEST NAME |
|
Lyme Disease Ab with Reflex to Blot (IgG, IgM) Includes: If Lyme Disease Antibody Screen is ≥0.90, then Lyme Disease Antibodies (IgG, IgM), Immunoblot will be performed at an additional charge (TC: 8593)(CPT code(s): 86617 (x2))
|
|
|
Test code |
94322 |
|
Preferred specimen |
3 mL whole blood collected in an EDTA (lavender-top) tube |
|
Turnaround time |
2-3 days |
Panel components:
|
TEST CODE |
TEST NAME |
|
Borrelia burgdorferi DNA, Qualitative, Real-Time PCR, Miscellaneousb |
|

|
|
Innovative testing options, ranging from routine to highly specialized tests |
|
|
Seamless EHR/IT integration with over 600 EHR systems |
|
|
Dedicated, national subject-matter experts are available for consultation and results interpretation |
|
|
Tools and services to optimize patient care |
|
|
One-stop convenience: ordering tests and supplies, scheduling specimen pickup, and obtaining test results with Quanum® Lab Services Manager |
|
|
Broad, in-network access with most major health plans |
|
|
Low out-of-pocket costs |
|
|
Payment Assistance Program for patients who are underinsured or uninsured |
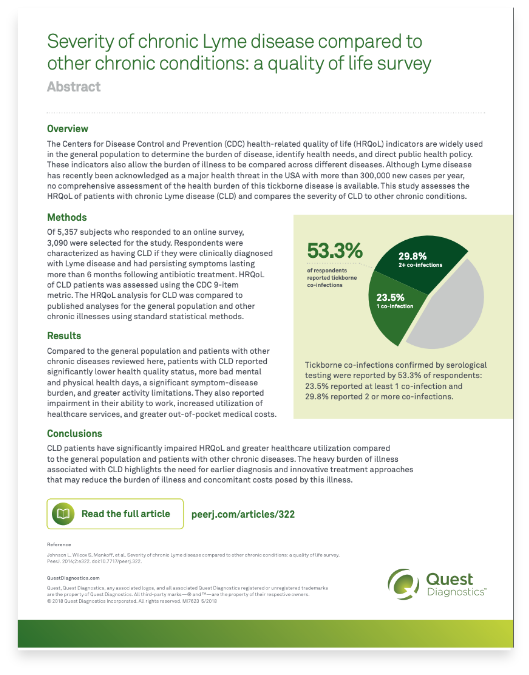
- Johnson L, Wilcox S, Mankoff J, et al. Severity of chronic Lyme disease compared to other chronic conditions: a quality of life survey. PeerJ. 2014;2:e322. doi: 10.7717/peerj.322
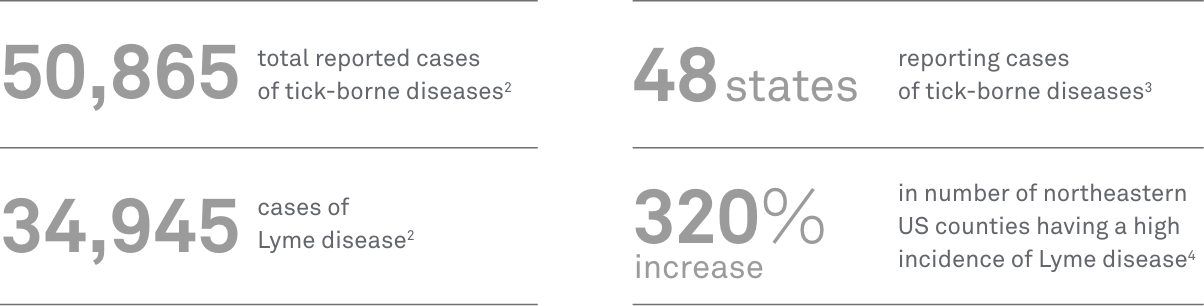
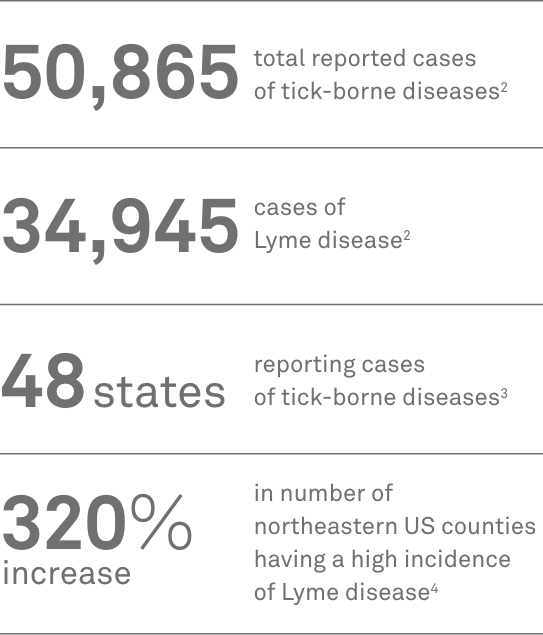
- CDC. Tickborne disease surveillance data summary. Reviewed August 11, 2022. Accessed February 24, 2023. https://www.cdc.gov/ticks/data-summary/index.html
- CDC. Overview of tickborne diseases. Reviewed August 5, 2022. Accessed February 24, 2023. https://www.cdc.gov/ticks/tickbornediseases/overview.html
- Kugeler KJ, Farley GM, Forrester JD, et al. Geographic distribution and expansion of human Lyme disease, United States. Emerg Infect Dis. 2015;21(8):1455-1457. doi:10.3201/eid2108.141878
- APHL. Suggested reporting language, interpretation and guidance regarding Lyme disease serologic test results. May 2021. Accessed February 3, 2023. https://www.aphl.org/aboutAPHL/publications/Documents/ID-2021-Lyme-Disease-Serologic-Testing-Reporting.pdf
- Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68(32):703. doi:10.15585/mmwr.mm6832a4
- CDC. Public Health Grand Rounds: Emerging tickborne diseases. Reviewed September 2019. Accessed March 3, 2023. https://www.cdc.gov/grand-rounds/pp/2017/20170321-tickborne-diseases.html